Hypsochromic shift (from ancient Greek ὕψος (upsos) 'height'; and χρῶμα chrōma, 'color') is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a shorter wavelength (higher frequency). Because the blue color in the visible spectrum has a shorter wavelength than most other colors, this effect is also commonly called a blue shift.
Hypsochromic Shift
This can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally related molecules in a substitution series can also show a hypsochromic shift. Hypsochromic shift is a phenomenon seen in molecular spectra, not atomic spectra - it is thus more common to speak of the movement of the peaks in the spectrum rather than lines.
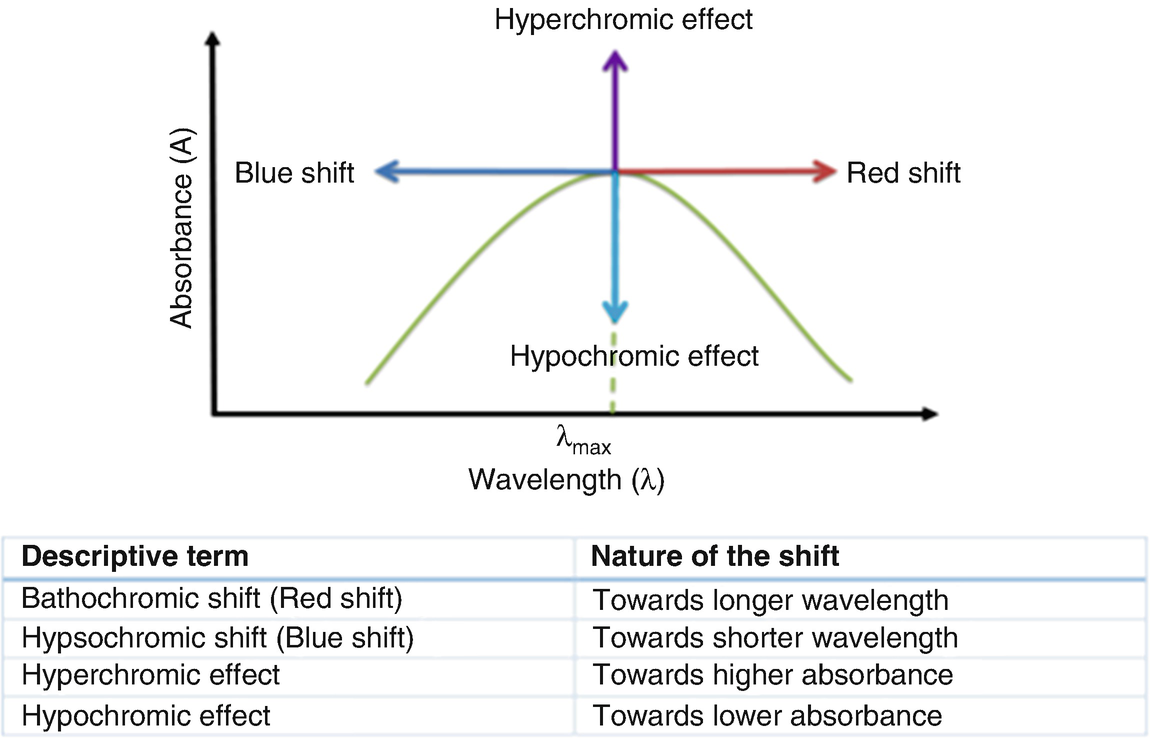
A hypsochromic shift occurs when the band position in a spectrum moves to shorter wavelength. This is sometimes called a blue shift. If a band moves in the opposite direction to higher wavelength or lower frequency, this is called a bathochromic shift. Definition of hypsochromic shift in the Definitions.net dictionary. Meaning of hypsochromic shift. What does hypsochromic shift mean? Information and translations of hypsochromic shift in the most comprehensive dictionary definitions resource on the web. A hypsochromic shift is the shift of a peak or signal to shorter wavelength (higher energy). Also called a blue shift. How does polarity affect absorption? As the solvents become more polar, the light absorbed by this dye shifts from the low energy, long wavelength (red) to the high energy, short wavelength (violet) end of the spectrum. Hypochromia means that the red blood cells have less color than normal when examined under a microscope. This usually occurs when there is not enough of the pigment that carries oxygen (hemoglobin) in the red blood cells. The most common cause of hypochromia in the United States is not enough iron in the body (iron deficiency).
- where is the wavelength of the spectral peak of interest and

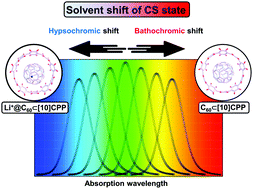
For example, β-acylpyrrole will show a hypsochromic shift of 30-40 nm in comparison with α-acylpyrroles.
See also[edit]
- Bathochromic shift, a change in band position to a longer wavelength (lower frequency).
Hypsochromic Shift Is Also Known As
Hypochromic Rbc Image
Hypsochromic Shift
